Experience with "Work Lights" used for Photography?
Jul 6, 2020 09:38:06 #
burkphoto wrote:
... fluorescent tubes, sodium vapor lamps, mercury... (show quote)
When I was working at UPittsburgh we had a stairway that was lit by low pressure sodium lamps. These lamps put out two spectral lines close together, making essentially a single color, yellow, that is probably more than 90% of the total energy output in the visible spectrum. In effect, when you entered the stairway everything was black and yellow. After a couple months my eyes got used to that and I started to see things in black and white. An interesting example of the brain's adaptability to the difference between expectation and reality.
However, our cameras and computer monitors don't have that adaptability and if I had taken a photo of the stairway, it would forever remain black and yellow (discounting the possibility of postprocessing, with which I could have made it black and white).
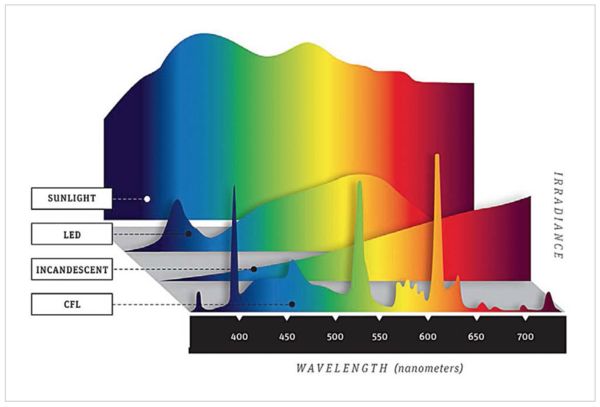
The spectra presented below show different light sources. These are approximations to reality since to some extent they will vary. The spectrum of sunlight for example will change depending on environmental conditions (think about red sunsets). Incandescent lamp spectra depend on the temperature. CFL spectra will depend on the phosphors used in the lamp. And LED spectra will depend on the specific semiconductor elements used in the LED (which is why LED lamps come in what they call "daylight", "cool white", "warm white", and "soft white").
But the point is that they all have different shapes. That means that there are colors in the LED spectrum that are significantly lower than the colors in sunlight, which has to be the standard against which we compare spectra, just because our eyes have evolved with only that spectrum to illuminate things. (Fire will be similar to incandescent lighting, but during most of our evolution, things were only illuminated by the sun). In particular, the figure below shows a dip in the LED spectrum just around 450nm.
That means there are colors that an LED lamp will not show well. OTOH, your camera detects and your computer monitor displays only 3 "colors". These colors are not narrow lines like the spikes you see in the CFL spectrum, but more like broad bumps in the spectrum. Essentially a color range. And they overlap. So the whole process is an approximation of an approximation.
.
https://www.led-professional.com/resources-1/articles/led-light-spectrum-enhancement-with-transparent-pigmented-glazes-by-light-spectrum-glazes/screen-shot-2016-06-20-at-15-39-21.png/@@images/4855fd0f-4ac8-4357-941d-2b1663773c39.png
(Download)
Jul 6, 2020 10:02:31 #
AKA A riddle wrapped in an enigma? Nice post, thank you. Now where is my ir sunglasses?
Jul 11, 2020 11:00:10 #
burkphoto wrote:
In practice, this is about what I've seen. The sun... (show quote)
Frequencies are missing!! Of course! Smack forehead. THAT explains why I can't get the WB right using some types of light. Thank you for that.
Maybe it also explains why I find LED lighting unpleasant to my eyes.
Jul 11, 2020 19:15:29 #
JD750 wrote:
Frequencies are missing!! Of course! Smack forehead. THAT explains why I can't get the WB right using some types of light. Thank you for that.
Maybe it also explains why I find LED lighting unpleasant to my eyes.
Maybe it also explains why I find LED lighting unpleasant to my eyes.
Quality of LED output varies WILDLY. Some are very accurate (and very expensive!).
If you want to reply, then register here. Registration is free and your account is created instantly, so you can post right away.


